Especially for an industry that needs minimal, medical practices, dentists, doctors, surgeons, hospitals, health clinics, pediatrics, psychiatrist, psychiatry, stomatology, chiropractor, veterinary clinics, and other medical-related practices 🏥👨⚕️🚑🔬
#webdesign #HTML5 #CSS3 #template #plugins #themes #WordPress #onepage #medical #responsive #retina #website #clinic #health #medic #clinical
Why most people are excluded from #clinical trials | Dr. Jarred Younger

052 - Why most people are excluded from clinical trials
#statstab #311 The analysis of continuous data from n-of-1 trials using paired cycles: a simple tutorial
Thoughts: @StephenSenn shows how to treat multiple #nof1 studies as a meta-analysis.
#sced #nof1 #metaanalysis #tutorial #clinical
https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-024-07964-7
The analysis of continuous data from n-of-1 trials using paired cycles: a simple tutorial - Trials
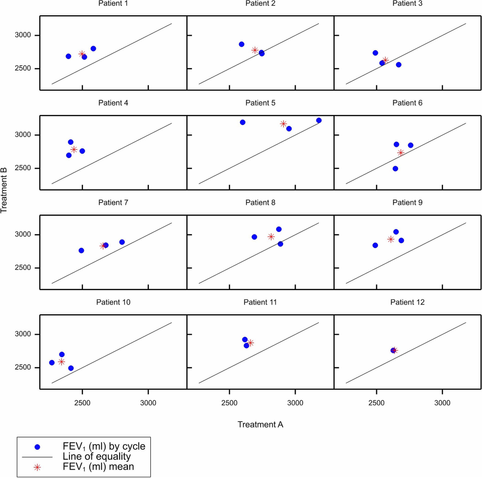
N-of-1 trials are defined and the popular paired cycle design is introduced, together with an explanation as to how suitable sequences may be constructed.Various approaches to analysing such trials are explained and illustrated using a simulated data set. It is explained how choosing an appropriate analysis depends on the question one wishes to answer. It is also shown that for a given question, various equivalent approaches to analysis can be found, a fact which may be exploited to expand the possible software routines that may be used.Sets of N-of-1 trials are analogous to sets of parallel group trials. This means that software for carrying out meta-analysis can be used to combine results from N-of-1 trials. In doing so, it is necessary to make one important change, however. Because degrees of freedom for estimating variances for individual subjects will be scarce, it is advisable to estimate local standard errors using pooled variances. How this may be done is explained and fixed and random effect approaches to combining results are illustrated.
#Tarot—Most #artistic, #engaging, #fun & #transformational #psychological tool that I use for +10yrs to revamp mindsets of #doctors, #lawyers, #engineers, #police, artists/#creatives & more worldwide; although I do not believe in #TarotCards as a prophetic/divinatory tool & use purely as a non-#clinical, non-#medical #Psychology tool I call #PsychologicalTarot.
Learn more on this use in one of my interviews entitled "Jung, The Tarot & Archetypes" (https://www.youtube.com/watch?v=F3qmSdfJOJs).

Jung, The Tarot & Archetypes
Trial concepts implemented across registers — ctrdata-trial-concepts
ctrdata includes (since version 1.21.0) functions that implement selected trial concepts. Concepts of clinical trials, such as their start or status of recruitment, require to analyse several fields against various pre-defined values. The structure and value sets of fields differ between all ctrdata-registers. In this situation, the implemented trial concepts simplify and accelerate a user's analysis workflow and also increase analysis consistency.
Especially for an industry that needs minimal, medical practices, dentists, doctors, surgeons, hospitals, health clinics, pediatrics, psychiatrist, psychiatry, stomatology, chiropractor, veterinary clinics, and other medical-related practices 🏥👨⚕️🚑🔬
#webdesign #HTML5 #CSS3 #template #plugins #themes #WordPress #onepage #medical #responsive #retina #website #clinic #health #medic #clinical