A federal judge ruled Medicare can negotiate prices for prescriptions this fall
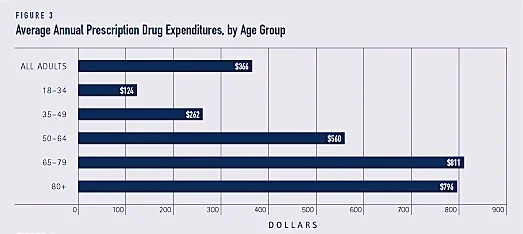
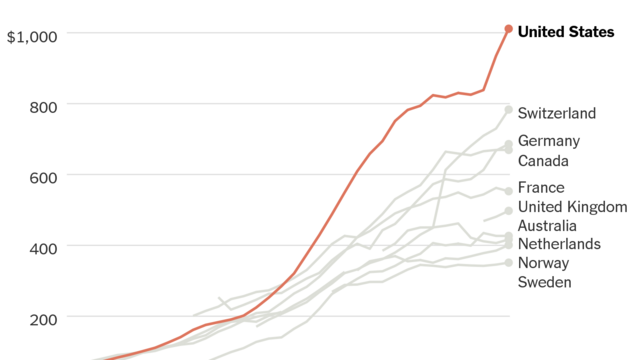
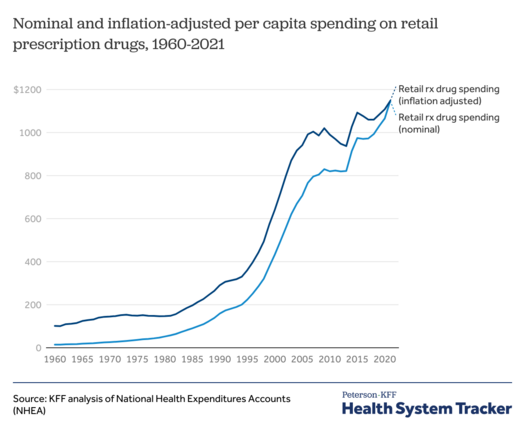
The US pays 2.4x the avg of industrialized nations for prescriptions
Brand name drugs make up 84% of total drug costs in the US—but account for only 8% of drugs sold
Tecartus +$25000 inc over 1yr
Fluconazole +1101% inc over 1yr
Lisinopril +539% inc over 1yr
Pfizer $10B revenue '22
Johnson & Johnson $95B revenue '22
You start working on a cure for high drug prices
We'll start working on a cure for MBAs