Need for awareness & surveillance of long-term #postCOVID neurodegenerative disorders. A position paper from the neuroCOVID‐19 task force of the European Academy of Neurology
https://link.springer.com/article/10.1007/s00415-025-13110-3
"An increase in the incidence of neurodegenerative diseases might be expected”
@longcovid
#LongCovid #PwLC #PostCovidSyndrome #LC #PASC #CovidBrain @covid19 #COVIDー19 #COVID19 #COVID #COVID_19 #SARSCoV2 @novid #novid @novid@a.gup.pe #CovidIsNotOver #auscovid19
Need for awareness and surveillance of long-term post-COVID neurodegenerative disorders. A position paper from the neuroCOVID‐19 task force of the European Academy of Neurology - Journal of Neurology
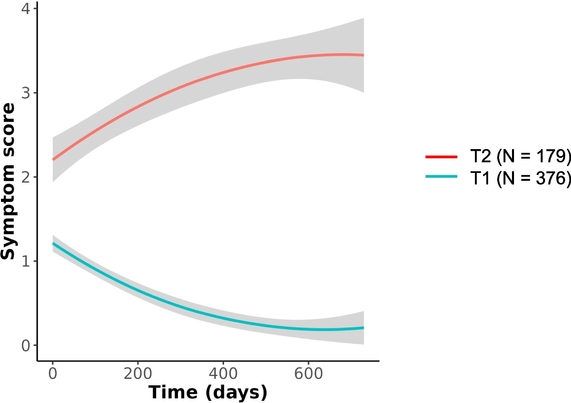
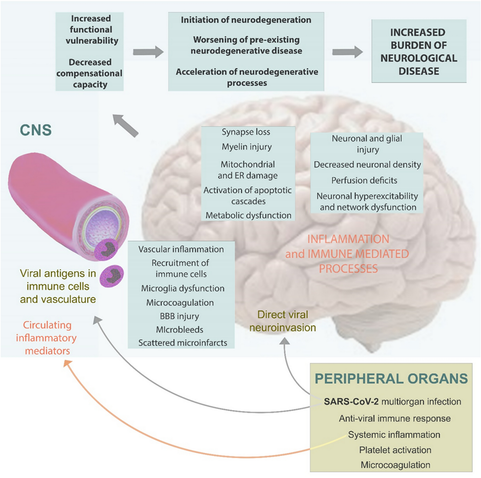
Background Neuropathological and clinical studies suggest that infection with SARS-CoV-2 may increase the long-term risk of neurodegeneration. Methods We provide a narrative overview of pathological and clinical observations justifying the implementation of a surveillance program to monitor changes in the incidence of neurodegenerative disorders in the years after COVID-19. Results Autopsy studies revealed diverse changes in the brain, including loss of vascular integrity, microthromboses, gliosis, demyelination, and neuronal- and glial injury and cell death, in both unvaccinated and vaccinated individuals irrespective of the severity of COVID-19. Recent data suggest that microglia play an important role in sustained COVID-19-related inflammation, which contributes to the etiology initiating a neurodegenerative cascade, to the worsening of pre-existing neurodegenerative disease or to the acceleration of neurodegenerative processes. Histopathological data have been supported by neuroimaging, and epidemiological studies also suggested a higher risk for neurodegenerative diseases after COVID-19. Conclusions Due to the high prevalence of COVID-19 during the pandemic, healthcare systems should be aware of, and be prepared for a potential increase in the incidence of neurodegenerative diseases in the upcoming years. Strategies may include follow-up of well-described cohorts, analyses of outcomes in COVID-19-registries, nationwide surveillance programs using record-linkage of ICD-10 diagnoses, and comparing the incidence of neurodegenerative disorders in the post-pandemic periods to values of the pre-pandemic years. Awareness and active surveillance are particularly needed, because diverse clinical manifestations due to earlier SARS-CoV-2 infections may no longer be quoted as post-COVID-19 symptoms, and hence, increasing incidence of neurodegenerative pathologies at the community level may remain unnoticed.