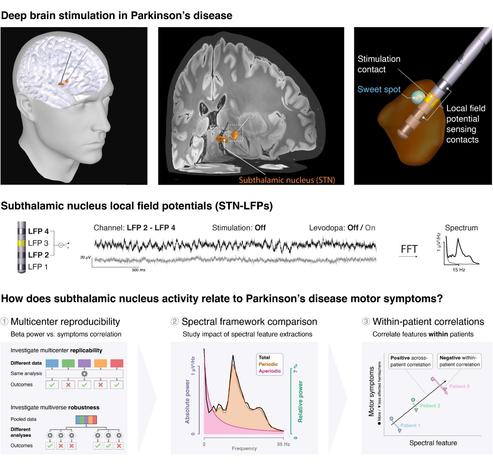
How does subthalamic nucleus activity relate to #Parkinson’s disease symptoms?
In a truly collaborative effort across deep brain stimulation centers, we aggregated a diverse dataset of invasive recordings from 119 patients.
#Reproducibility analysis:
↳ Levodopa modulates theta more strongly than beta oscillations.
↳ Beta does not consistently correlate with symptoms in single datasets.
↳ Replicating previous correlations requires cohorts of over 100 patients.
#Powerspectrum analysis:
↳ Absolute power provides clearer insights than normalized power.
↳ Separating oscillations from the broadband improves interpretability.
↳ Theta & Low β & Low 𝛄 explained symptom severity better than beta alone.
#Aperiodic broadband power:
↳ Likely indicates neuronal spiking.
↳ Reflects symptom severity within patients.
↳ Shows promise as a biomarker for adaptive deep brain stimulation.
Full article: https://doi.org/10.1101/2025.03.11.642600
Beyond beta rhythms: Aperiodic broadband power reflects Parkinson’s disease severity–a multicenter study
Parkinson’s disease is linked to increased beta oscillations in the subthalamic nucleus, which correlate with motor symptoms. However, findings across studies have varied. Our standardized analysis of multicenter datasets shows that small sample sizes contributed to these discrepancies—a challenge we address by pooling datasets into one large cohort (n=119). Moving beyond beta power, we disentangled spectral components reflecting distinct neural processes. Combining aperiodic offset, low beta, and low gamma oscillations explained significantly more variance in symptom severity than beta alone. Moreover, interhemispheric within-patient analyses showed that, unlike beta oscillations, aperiodic broadband power–likely reflecting spiking activity–was increased in the more affected hemisphere. These findings identify aperiodic broadband power as a potential biomarker for adaptive deep brain stimulation and provide novel insights into the relationship between subthalamic hyperactivity and motor symptoms in human Parkinson’s disease. ### Competing Interest Statement K.A. received educational grants from Medtronic and Abbott. WJN received honoraria for consulting from InBrain - Neuroelectronics that is a neurotechnology company and honoraria for talks from Medtronic that is a manufacturer of deep brain stimulation devices unrelated to this manuscript. LKF received honoraria for talks from Medtronic. A.A.K. has served on advisory boards of Medtronic and has received honoraria and travel support from Medtronic and Boston Scientific. A.A.K. reports a relationship with Medtronic that includes consulting or advisory, speaking and lecture fees, and travel reimbursement. A.A.K. reports a relationship with Boston Scientific Corporation that includes speaking and lecture fees, and travel reimbursement. P.K. has served on advisory boards for MedTronic, AbbVie and Gerresheimer and received lecture fees from Stadapharm and AbbVie. The remaining authors declare no competing interests.