https://doi.org/doi:10.17912/micropub.biology.001764
https://pubmed.ncbi.nlm.nih.gov/41050330/
#Myosin
Hypertrophic (HCM) and dilated (DCM) cardiomyopathy variants in genes encoding the myosin heavy chain (MYH7), myosin light chains (MYL2 and MYL3), and cardiac myosin binding protein-C (cMyBP-C, MYBPC3) lead to cardiac hypertrophy or dilatation, with abnormal contractility, relaxation, and energy consumption. Here we defined the structural consequences of >200 pathogenic and benign missense variants in these genes by mapping variants onto a cryo-EM-based atomic model of the human cardiac thick filament. We identified HCM variants residing in 31 molecular interfaces of the complex thick filament interactome, including the two main interfaces of the myosin interacting-heads motif (IHM), and interfaces involving the myosin heavy chain, essential and regulatory light chains, and cMyBP-C. Pathogenic DCM missense variants are rare, and altered only interfaces involving the myosin IHM and tails. None of the 21 variants classified as benign were within interfaces. We demonstrate earlier disease onset and adverse outcomes in HCM patients with pathogenic variants within versus outside of molecular interfaces, emphasizing their importance in normal thick filament function and improving risk stratification of patients. The dissimilar distribution of DCM and HCM variants could explain the different features of the two phenotypes. ### Competing Interest Statement Christine E. Seidman reports the following interests: Scientific advisory Tenaya therapeutics; Board of directors, Burroughs Wellcome Fund US and Merck). None of the interests had a role in any aspect of this study. The other authors have declared that no conflict of interest exists. National Institutes of Health, https://ror.org/01cwqze88, NHLBI HL164560, NIAMS AR081941, NHLBI K08HL164885 British Heart Foundation, BBC/F/21/220106 National Science Foundation (NSF) Engineering Research Center on Cellular Metamaterials, EEC-1647837
Desmoglein-2 (DSG2), a critical component of the cardiac desmosome and located at the cardiomyocyte-cardiomyocyte intercalated disc, is essential for cell-cell adhesion, cardiomyocyte mechanical stability, and electrical coupling between cells. However, its relative contribution in maintaining cardiac function at the sarcomere level remains unclear. Using 4-week-old (adolescent) and 16-week-old (adult) homozygous knock-in Dsg2-mutant (Dsg2mut/mut) mice, we found that loss of DSG2 leads to early onset chamber- and age-dependent cardiac dysfunction driven by Z-disc structural defects and increased myosin detachment rate. Interestingly, Ca2+;-activated force was markedly reduced in adolescent Dsg2mut/mut permeabilized left ventricular cardiac muscle bundles but preserved in permeabilized isolated cardiomyocytes. This disparity demonstrates that DSG2 is not only crucial for mechanical coupling between cardiomyocytes but also for force transmission within and between sarcomeres, revealing a novel role for DSG2 in maintaining contractile integrity at both the cellular and tissue levels. ### Competing Interest Statement The authors have declared no competing interest. American Heart Association, 19CDA34760185 National Institutes of Health, https://ror.org/01cwqze88, P30 GM138395 U.S. National Science Foundation, DMR-1829070 National Institutes of Health, https://ror.org/01cwqze88, 1-P30--GM124166--01A1 British Heart Foundation, 222567/Z/21/Z
Toxoplasma gondii ( T. gondii ) is a single-celled Apicomplexan parasite that relies on a highly polarized endomembrane system for its invasion into and survival within host cells. Recent advancements in imaging technologies have revealed that vesicle transport and organization of organelles in the endomembrane pathway requires a highly dynamic actin cytoskeleton. These dynamics in turn rely on the activity of Myosin F (MyoF), a molecular motor unique to Alveolates. The defining characteristic of this molecular motor is a WD40 beta-propeller domain, exclusively found in this class of myosin. To understand the mechanism by which MyoF controls the dynamics and organization of actin, we studied the biophysical properties of the purified motor in vitro. A MyoF construct lacking its WD40 tail domain (MyoFΔtail) is dimeric and can bind and translocate actin in an in vitro motility assay. Single molecule studies show that the dimeric construct is non-processive however small ensembles move inefficiently on single filaments of skeletal actin. In contrast, single molecules of the full-length motor move processively on Toxoplasma actin and jasplakinolide-stabilized skeletal actin bundles. Electron microscopy of negatively stained images of MyoF and quantitative size exclusion chromatography shows that the WD40 domain oligomerizes to form a complex containing multiple dimeric molecules, which provides an explanation for why the full-length motor is processive compared to the dimeric MyoFΔtail construct. Finally, we show that MyoF binds microtubules through its WD40 domain and can slide actin filaments relative to microtubules. We propose a model whereby MyoF oligomers drive actin dynamics by translocating filaments relative to the parasites cytoskeleton. These molecule features provide new insight into how MyoF functions in the cell to regulate actin organization during vesicle transport. ### Competing Interest Statement The authors have declared no competing interest. National Institutes of General Medical Science, R35GM138316, R35GM138316-02S1
Toxoplasma gondii ( T. gondii ) is a single-celled Apicomplexan parasite that relies on a highly polarized endomembrane system for its invasion into and survival within host cells. Recent advancements in imaging technologies have revealed that vesicle transport and organization of organelles in the endomembrane pathway requires a highly dynamic actin cytoskeleton. These dynamics in turn rely on the activity of Myosin F (MyoF), a molecular motor unique to Alveolates. The defining characteristic of this molecular motor is a WD40 beta-propeller domain, exclusively found in this class of myosin. To understand the mechanism by which MyoF controls the dynamics and organization of actin, we studied the biophysical properties of the purified motor in vitro. A MyoF construct lacking its WD40 tail domain (MyoFΔtail) is dimeric and can bind and translocate actin in an in vitro motility assay. Single molecule studies show that the dimeric construct is non-processive however small ensembles move inefficiently on single filaments of skeletal actin. In contrast, single molecules of the full-length motor move processively on Toxoplasma actin and jasplakinolide-stabilized skeletal actin bundles. Electron microscopy of negatively stained images of MyoF and quantitative size exclusion chromatography shows that the WD40 domain oligomerizes to form a complex containing multiple dimeric molecules, which provides an explanation for why the full-length motor is processive compared to the dimeric MyoFΔtail construct. Finally, we show that MyoF binds microtubules through its WD40 domain and can slide actin filaments relative to microtubules. We propose a model whereby MyoF oligomers drive actin dynamics by translocating filaments relative to the parasites cytoskeleton. These molecule features provide new insight into how MyoF functions in the cell to regulate actin organization during vesicle transport. ### Competing Interest Statement The authors have declared no competing interest. National Institutes of General Medical Science, R35GM138316, R35GM138316-02S1
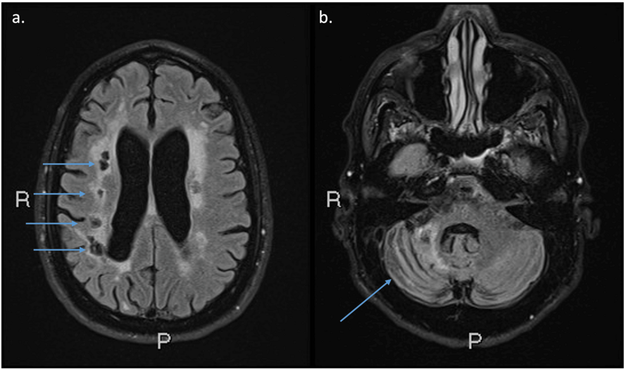
We present a patient with complex cerebral and arterial pathology found to have a rare heterozygous myosin heavy chain 11 (MYH11) missense variant. A 58-year-old male was seen after recurrent strokes at ages 43, 52, and 55. Brain magnetic resonance angiography (MRA) showed five outpouchings or small aneurysms arising from the intracranial internal carotid arteries (ICAs), a larger basilar artery aneurysm, and severely stenotic segments of several intracranial arteries. Brain magnetic resonance imaging (MRI) showed a mixture of small and large artery old infarcts and white matter disease. The descending thoracic aorta and the abdominal aorta were both aneurysmal on computerized tomography angiography. Whole exome sequencing (WES) identified a rare heterozygous MYH11: c.3818G>T, p.(Arg1273Leu) missense variant. This case report highlights the risk for vascular abnormalities, including cerebrovascular disease, in MYH11 pathogenic variants and underscores the importance of vigilant monitoring and early prophylactic interventions for stroke prevention in this patient population.
Molecular organization of the plasma membrane (PM) at nano and micron scales is critical for its function in all living cells. This emerges not only from the self-assembly of lipids and proteins but also from active forces originating in the underlying cytoskeletal cortex. These forces drive membrane molecules into non-equilibrium steady state patterns such as nanoclusters. However, the molecular agents connecting membrane organization with cytoskeletal dynamics and stresses have remained unknown. Here we show that two classes of ubiquitous ancestral non-muscle myosins are deployed for the organization of different types of membrane components. Inner-leaflet localized Class I myosins link outer-leaflet GPI-anchored molecules to juxta-membrane actin-filaments, whereas the more cortically-localized Class II myosins operate on transmembrane proteins endowed with actin-binding capacity. Consistent with an active Flory Huggins theory for phase separation, these observations show that the distinct motor-driven membrane molecules generate spatially segregated mesoscale domains, enriched in nanoclusters derived from different myosin classes. Moreover, chemically reversible post-translational modifications such as palmitoylation enable concatenation of these domains by enhancing affinity of the membrane domain constituents for each other. We anticipate that the segregation potential of the ATP-fueled cell membrane is made available for the crucial purpose of modulating information transduction because it can be regulated in space and time during the construction of signaling cascades, underpinning functional plasma membrane organization. ### Competing Interest Statement The authors have declared no competing interest. CSIR, MLP5005 EMBO, ALTF 1519-2013 Fundación General CSIC's ComFuturo programme, European Union Horizon 2020 /Marie Skłodowska-Curie grant agreement No.101034263 CSIR NET JRF UGC NET JRF Department of Biotechnology Wellcome Trust India Alliance Margadarshi Fellowship, IA/M/15/1/502018 Leverhulme Trust, UK, LIP- 40 2021-017 Department of Atomic Energy, https://ror.org/02m388s04, RTI 4006 JC Bose National Fellowship, Department of Science and Technology, India, JBR/2021/000014, JCB/2018/00030, Simons Foundation, 287975